Better Inform
Biopsy Decisions
IsoPSA provides improved risk stratification for high-grade prostate cancer.
The proof is in the PSA protein structure
IsoPSA helps determine if elevated PSA came from cancer cells.
IsoPSA analyzes the structure of the PSA protein, providing more biological certainty around whether an elevated PSA is due to cancer or not.
Better identify patients who may benefit from a biopsy
IsoPSA demonstrates a higher specificity for csPCa than the standard of care:
IsoPSA specificity for csPCa
Klein, et al., Urol Oncol. 2022
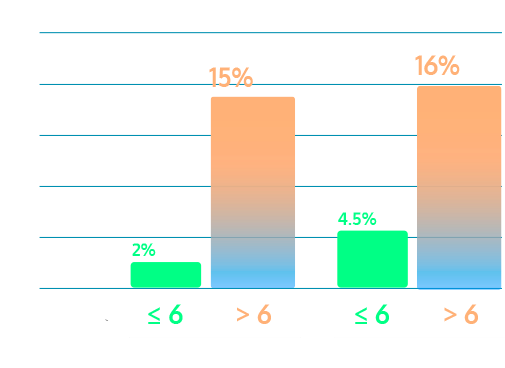
IsoPSA is predictive of risk for csPCa over time
A single result accurately predicts csPCa over 2.5 years.
IsoPSA Index ≤ 6
Demonstrated low risk of csPCa, providing confidence to defer biopsy or extend intervals between biopsies.
IsoPSA Index > 6
Nearly 50% develop csPCa within 2.5 years (30 months), indicating closer monitoring and follow-up is warranted.

Abdallah, et al., Urology. 2025
Enhance biopsy decision-making with or without MRI
IsoPSA improves the interpretation of PI-RADS scores.
IsoPSA can help:
- Clarify equivocal MRI (PI-RADS 3) to better triage patients.
- Identify risk of MRI-invisible csPCa in PI-RADS 1-2 results.
Additionally, IsoPSA results can refine the biopsy decision-making process when MRI is not an option.
Benidir, et al., Urology. 2023
Easily incorporated into your current clinical workflow
Only a blood sample is needed; no additional patient information is required
Results are not affected by BPH medications, exercise, or sexual activity
Validated and proven effective for repeat biopsy and biopsy-naïve patients
No digital rectal exam (DRE) required
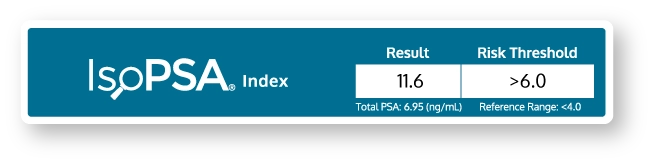
IsoPSA provides a simple report to facilitate better patient counseling.
The report includes a total PSA result, eliminating the need order a separate repeat PSA test.
1. Klein, et al., Urol Oncol. 2022 Sep;40(9):408.e9-408.e18.
2. Abdallah, et al., Urology Jan15:S0090-4295(25)00031-7.
3. Benidir et al., Urology. 2023 Jun;176:115-120